Review Paths
Review Path Flowchart (PDF)
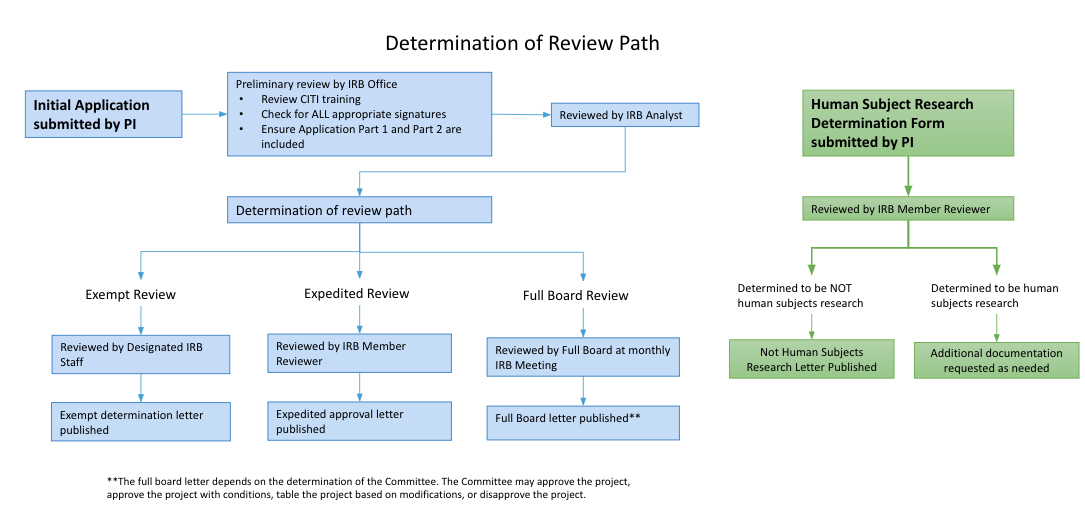
Human Subject Research Determination
If you are unsure if your project requires IRB review and approval, please download, complete, and submit the HSRD Form through IRBNet. You will receive an official determination confirming no IRB Approval is needed or additional information will be requested.
Exempt Review
To receive an Exempt Determination from the IRB, a protocol must fall into one or more of eight (8) federally-defined exempt categories. Examples of Exempt research are: anonymous surveys/interviews, passive observation of public behavior without collection of identifiable information, retrospective chart/record/data reviews, analysis of discarded pathological specimens without identifiers, etc.
IRB protocols that have received an Exempt Determination do not require Continuing Review.
Expedited Review
To qualify for an Expedited Review, research should fall into one or more of nine (9) federally-defined Expedited categories and present no greater than minimal risk to participants.
Full IRB Review
Protocols that do not meet either Exempt or Expedited review criteria will be added to the next available agenda for review at the fully convened IRB Meeting. Research activities presenting greater than minimal risk or transactions increasing the potential risk will always be referred to a fully convened IRB Meeting for review.
Protocols reviewed by the fully convened IRB will be approved for up to one year. If you plan to continue obtaining data from human subjects or collecting or analyzing identifiable private information for your research project, a Continuing Review application must be reviewed and approved by the IRB prior to the Expiration Date.
The IRB recommends that Continuing Review Applications be submitted to the IRB Office 30-45 days in advance of the Expiration Date of the protocol. This will allow appropriate time for review, administrative modifications, and approval prior to protocol expiration.
Please Note: If the Continuing Review Application is submitted prior to the Expiration Date, this does not guarantee the application will receive IRB Approval before the protocol expires (i.e. if the Continuing Review has been submitted shortly before expiration). If the protocol does expire after a Continuing Review Application has been submitted, all research activities involving human subjects must cease until approval is granted. If a Continuing Review Application is not received by the IRB prior to the Expiration Date, the protocol will expire and a new Initial Protocol Application must be submitted and approved in order to continue human subject research activities.